Your Why is soap used as a cleansing agent images are available. Why is soap used as a cleansing agent are a topic that is being searched for and liked by netizens today. You can Get the Why is soap used as a cleansing agent files here. Find and Download all royalty-free images.
If you’re looking for why is soap used as a cleansing agent images information connected with to the why is soap used as a cleansing agent keyword, you have come to the ideal site. Our website frequently provides you with hints for refferencing the highest quality video and image content, please kindly hunt and locate more informative video articles and images that match your interests.
Why Is Soap Used As A Cleansing Agent. Soap on the other hand is a different substance used with water meant for washing and cleaning. Why is soap solution a better cleansing agent than ordinary water. For the most effective hand washing you must use soap and you must be thorough. Soap is a fatty acid of a salt.
 Detergents Used For Cleaning Of Pharmaceutical Equipments Pharmaceutical Guidelines Cleaning Detergents Dish Soap Bottle From pinterest.com
Detergents Used For Cleaning Of Pharmaceutical Equipments Pharmaceutical Guidelines Cleaning Detergents Dish Soap Bottle From pinterest.com
It acts as a cleansing agent and helps in removing dirt and grease. In industrial settings soaps are used as thickeners components of some lubricants and precursors to catalysts. Why is soap solution a better cleansing agent than ordinary water. Soaps are good cleaning agents in soft water but their cleaning action is drastically reduced in hard water which contains Ca2 and Mg2 ionsThese ions react with soap to form Insoluble salts of calcium and magnesium called scumwhich reduces the effectiveness of the soap and the soap gets wastedOn the other hand Detergents do not form these compounds because they are made from propene. Soap contains base in it. This ability is due to the structure of soaps and detergents.
In high concentrations its a significant skin sensitizer.
Soaps are not effective cleansing agent in hard water because hard water contains dissolved calcium and magnesium ions which reacts with soap to form an insoluble substance. Also known as lye sodium hydroxide is a highly alkaline ingredient used in small amounts in cosmetics to establish and hold the pH of a product. Work up a lather because the friction helps lift dirt and oils from your skin according to the Centers for Disease. It acts as a cleansing agent and helps in removing dirt and grease. Soap is essentially a cleansing agent created by the chemical reaction of a fatty acid with an alkali metal hydroxide. This ability is due to the structure of soaps and detergents.
 Source: id.pinterest.com
Source: id.pinterest.com
Hence a lot of soap is wasted in hard water. They are used for cleaning purposes and are sodium or potassium salts of long-chain fatty acids eg stearic oleic and palmitic acids. It acts as a cleansing agent and helps in removing dirt and grease. Soap contains base in it. In high concentrations its a significant skin sensitizer.
 Source: slideshare.net
Source: slideshare.net
These help in the removal of fats that bind other materials to the fabric or skin. It can surround oil making it easier to rinse it away with water. Soaps are used as cleansers and lubricants. Also to know is sodium hydroxide safe to use in soap. Used as cleansing agent which combines with impurities and dirt to make them more water soluble which is better for washing clothes and dishes.
 Source: pinterest.com
Source: pinterest.com
In a domestic setting soaps are su rfactants usually used for washing bathing and other types of housekeeping. Soaps are not effective cleansing agent in hard water because hard water contains dissolved calcium and magnesium ions which reacts with soap to form an insoluble substance. Soap is used as a cleansing agent because it aids in the removal of germs through its sudsy action and alkali content. The cleaning agent in the orange cleaners is called D-limonene. Soaps are used as cleansers and lubricants.
 Source: co.pinterest.com
Source: co.pinterest.com
In a domestic setting soaps are su rfactants usually used for washing bathing and other types of housekeeping. Also known as lye sodium hydroxide is a highly alkaline ingredient used in small amounts in cosmetics to establish and hold the pH of a product. The process is known as saponification. Soap is essentially a cleansing agent created by the chemical reaction of a fatty acid with an alkali metal hydroxide. This ability is due to the structure of soaps and detergents.
 Source: id.pinterest.com
Source: id.pinterest.com
Used as cleansing agent which combines with impurities and dirt to make them more water soluble which is better for washing clothes and dishes. In industrial settings soaps are used as thickeners components of some lubricants and precursors to catalysts. The cleansing action of soaps and detergents. It is a powerful cleaner used in everything from hand soap to engine degreasers. Why is soap used as a cleaning agent.
 Source: pinterest.com
Source: pinterest.com
Soap on the other hand is a different substance used with water meant for washing and cleaning. In industrial settings soaps are used as thickeners components of some lubricants and precursors to catalysts. Soap is used as a cleansing agent because it aids in the removal of germs through its sudsy action and alkali content. In high concentrations its a significant skin sensitizer. Soaps are good cleaning agents in soft water but their cleaning action is drastically reduced in hard water which contains Ca2 and Mg2 ionsThese ions react with soap to form Insoluble salts of calcium and magnesium called scumwhich reduces the effectiveness of the soap and the soap gets wastedOn the other hand Detergents do not form these compounds because they are made from propene.
 Source: pinterest.com
Source: pinterest.com
Soap is a salt of a fatty acid used in a variety of cleansing and lubricating products. Soaps are good cleaning agents in soft water but their cleaning action is drastically reduced in hard water which contains Ca2 and Mg2 ionsThese ions react with soap to form Insoluble salts of calcium and magnesium called scumwhich reduces the effectiveness of the soap and the soap gets wastedOn the other hand Detergents do not form these compounds because they are made from propene. These help in the removal of fats that bind other materials to the fabric or skin. Hence a lot of soap is wasted in hard water. In high concentrations its a significant skin sensitizer.
 Source: pinterest.com
Source: pinterest.com
Soap is a fatty acid of a salt. It acts as a cleansing agent and helps in removing dirt and grease. Soaps are generally made by reacting an alkali like sodium hydroxide in liquid form with naturally occurring fats or fatty acids produced from animals and plants. In high concentrations its a significant skin sensitizer. Soap on the other hand is a different substance used with water meant for washing and cleaning.
 Source: ar.pinterest.com
Source: ar.pinterest.com
Soaps are good cleaning agents in soft water but their cleaning action is drastically reduced in hard water which contains Ca2 and Mg2 ionsThese ions react with soap to form Insoluble salts of calcium and magnesium called scumwhich reduces the effectiveness of the soap and the soap gets wastedOn the other hand Detergents do not form these compounds because they are made from propene. Soap on the other hand is a different substance used with water meant for washing and cleaning. Also to know is sodium hydroxide safe to use in soap. It is a powerful cleaner used in everything from hand soap to engine degreasers. Put more simply its made by mixing fats and oils with a base for example.
 Source: pinterest.com
Source: pinterest.com
Also known as lye sodium hydroxide is a highly alkaline ingredient used in small amounts in cosmetics to establish and hold the pH of a product. The process is known as saponification. Soap is used as a cleansing agent because it aids in the removal of germs through its sudsy action and alkali content. The cleansing action of soaps and detergents. Soap cleans by acting as a surfactant and emulsifier.
 Source: pinterest.com
Source: pinterest.com
These help in the removal of fats that bind other materials to the fabric or skin. Soaps are not effective cleansing agent in hard water because hard water contains dissolved calcium and magnesium ions which reacts with soap to form an insoluble substance. Also to know is sodium hydroxide safe to use in soap. Mostly made from a compound of natural oils or fats with sodium hydroxide or another strong alkali. Soap is used as a cleansing agent because it aids in the removal of germs through its sudsy action and alkali content.
 Source: de.pinterest.com
Source: de.pinterest.com
In a domestic setting soaps are su rfactants usually used for washing bathing and other types of housekeeping. For the most effective hand washing you must use soap and you must be thorough. The cleansing action of soaps and detergents. In industrial settings soaps are used as thickeners components of some lubricants and precursors to catalysts. Soap cleans by acting as a surfactant and emulsifier.
 Source: pinterest.com
Source: pinterest.com
Soap is used as a cleansing agent because it aids in the removal of germs through its sudsy action and alkali content. Why is a soap solution a better cleaning agent than ordinary water. The process is known as saponification. In a domestic setting soaps are su rfactants usually used for washing bathing and other types of housekeeping. The cleaning agent in the orange cleaners is called D-limonene.
 Source: ro.pinterest.com
Source: ro.pinterest.com
Soaps are a common detergent. The cleaning agent in the orange cleaners is called D-limonene. Hence a lot of soap is wasted in hard water. Because it has ingredients in it that will get more Germs off your skin than ordinary water. The process is known as saponification.
 Source: pinterest.com
Source: pinterest.com
The cleaning agent in the orange cleaners is called D-limonene. Soap is a fatty acid of a salt. Used as cleansing agent which combines with impurities and dirt to make them more water soluble which is better for washing clothes and dishes. Soaps are generally made by reacting an alkali like sodium hydroxide in liquid form with naturally occurring fats or fatty acids produced from animals and plants. Soap is used as a cleansing agent because it aids in the removal of germs through its sudsy action and alkali content.
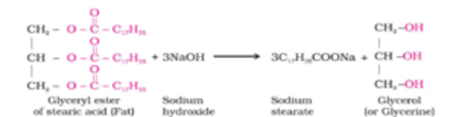 Source: thefactfactor.com
Source: thefactfactor.com
Mostly made from a compound of natural oils or fats with sodium hydroxide or another strong alkali. Soaps are not effective cleansing agent in hard water because hard water contains dissolved calcium and magnesium ions which reacts with soap to form an insoluble substance. Soap is used as a cleansing agent because it aids in the removal of germs through its sudsy action and alkali content. Soap contains base in it. Also known as lye sodium hydroxide is a highly alkaline ingredient used in small amounts in cosmetics to establish and hold the pH of a product.
 Source: onelearningsolution.blogspot.com
Source: onelearningsolution.blogspot.com
So use soap when you wash your hands. Soaps are not effective cleansing agent in hard water because hard water contains dissolved calcium and magnesium ions which reacts with soap to form an insoluble substance. Why is a soap solution a better cleaning agent than ordinary water. For the most effective hand washing you must use soap and you must be thorough. Soap is essentially a cleansing agent created by the chemical reaction of a fatty acid with an alkali metal hydroxide.
 Source: pinterest.com
Source: pinterest.com
Soap on the other hand is a different substance used with water meant for washing and cleaning. Hence a lot of soap is wasted in hard water. Put more simply its made by mixing fats and oils with a base for example. Soap is essentially a cleansing agent created by the chemical reaction of a fatty acid with an alkali metal hydroxide. The process is known as saponification.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title why is soap used as a cleansing agent by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





