Your Which statement about niels bohrs atomic model is true images are ready. Which statement about niels bohrs atomic model is true are a topic that is being searched for and liked by netizens now. You can Download the Which statement about niels bohrs atomic model is true files here. Find and Download all free photos.
If you’re searching for which statement about niels bohrs atomic model is true images information related to the which statement about niels bohrs atomic model is true interest, you have pay a visit to the right blog. Our website frequently provides you with suggestions for seeking the highest quality video and picture content, please kindly search and locate more enlightening video content and graphics that match your interests.
Which Statement About Niels Bohrs Atomic Model Is True. Bohrs model had one significant drawback - they were only a 2-D diagram of the truth. In plain words Bohrs model was a 2-D shape while Sommerfields was a 3-D shape of an atom. It contradicted the Heisenberg uncertainty principle. Which statement about Niels Bohrs atomic model is true.
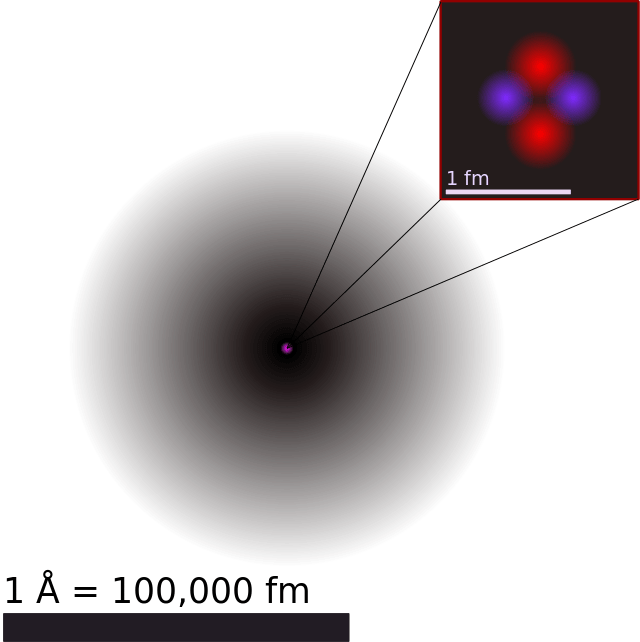 What Is Bohr S Atomic Model Universe Today From universetoday.com
What Is Bohr S Atomic Model Universe Today From universetoday.com
B concentrated in the center of an atom. Kumar is producing the photoelectric effect by using red light. D There is a dense positively charged mass in the center of an atom. Each orbit has a specific energy level. Bohrs most important work was developing a model of the atom. Which feature of Bohrs atomic model is no longer accepted as true by todays scientists.
B concentrated in the center of an atom.
Bohr model description of the structure of atoms especially that of hydrogen proposed 1913 by the Danish physicist Niels BohrThe Bohr model of the atom a radical departure from earlier classical descriptions was the first that incorporated quantum theory and was the predecessor of wholly quantum-mechanical models. D concentrated at multiple sites in an atom. According to the Bohr model of an atom what happens when an electron moves from the second energy level to the third energy level and then back to the second energy level. In an atomic model that includes a nucleus positive charge is. C spread evenly throughout an atom. The difference between the energies of those orbits would be equal to the energy of the photon.
 Source: researchgate.net
Source: researchgate.net
In reality the actual model of the atom is the Sommerfield atom where we see electrons travelling in an elliptical path around the nucleus but in the xyz plane. D There is a dense positively charged mass in the center of an atom. Bohrs model of the structure of an atom includes ideas from both classical and quantum physics asked Sep 24 2016 in Physics Space Science by Pinchtour introductory-physics. According to Niels Bohrs atomic model what occurs when an atom absorbs radiated energy. Each orbit has a specific energy level.
 Source: sites.google.com
Source: sites.google.com
Bohr atomic model and the models after that explain the properties of atomic electrons on the basis of certain allowed possible values. In reality the actual model of the atom is the Sommerfield atom where we see electrons travelling in an elliptical path around the nucleus but in the xyz plane. Kumar is producing the photoelectric effect by using red light. An electron shifts from a lower energy level to a higher one. Orbits close to the nucleus have no energy.
 Source: researchgate.net
Source: researchgate.net
D concentrated at multiple sites in an atom. Each orbit has a specific energy level. Bohrs model of the structure of an atom includes ideas from both classical and quantum physics asked Sep 24 2016 in Physics Space Science by Pinchtour introductory-physics. Energy is absorbed and then released to form an emission line. Option C It explained the odds of finding the position of an electron.
 Source: pinterest.com
Source: pinterest.com
It is the modification of the Rutherford Model. A located in the space outside the nucleus. Answers many questions about atoms. Asked Jun 26 2017 in Chemistry by LittleBuddha. Niel Bohrs Atomic Theory states that an atom is like a planetary model where electrons were situated in discretely energized orbits.
 Source: electrical4u.com
Source: electrical4u.com
Thomsons discovery of the electron in 1897 scientists were aware that the atom was made up of smaller particles. B concentrated in the center of an atom. A located in the space outside the nucleus. Niels Bohr was a Danish physicist born in Copenhagen on October 7 1885. Answers many questions about atoms.
 Source: universetoday.com
Source: universetoday.com
D There is a dense positively charged mass in the center of an atom. Niels Bohr was a Danish physicist born in Copenhagen on October 7 1885. The energy of the electron in a given orbit is not fixed. According to the Bohr model of an atom what happens when an electron moves from the second energy level to the third energy level and then back to the second energy level. The diagram shows Niels Bohrs model of an atom.
 Source: expii.com
Source: expii.com
Higher orbits have lower energies. Each orbit has a specific energy level. Bohrs model of the structure of an atom includes ideas from both classical and quantum physics asked Sep 24 2016 in Physics Space Science by Pinchtour introductory-physics. Higher orbits have lower energies. Electrons can exist in any energy level.
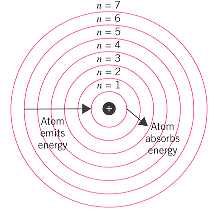 Source: chemed.chem.purdue.edu
Source: chemed.chem.purdue.edu
D There is a dense positively charged mass in the center of an atom. Electrons can exist in any energy level. In an atomic model that includes a nucleus positive charge is. Answers many questions about atoms. A Most of the space in an atom is empty b The total number of neutrons and protons is always equal in a neutral atom c The total number of electrons and protons in an atom is always equal d The total number f electrons in any energy level can be calculated by the formula 2n2.
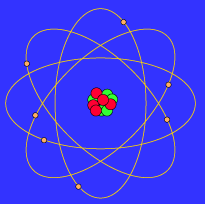 Source: pas.rochester.edu
Source: pas.rochester.edu
The atom would radiate a photon when an excited electron would jump down from a higher orbit to a lower orbit. Bohr was able to predict the difference in energy between each energy level allowing us to predict the energies of each line in the emission spectrum of hydrogen and understand why electron energies are quantized. An atom is held together by. The main problem with Bohrs model is that it works very well for atoms with only one electron like H or He but not at all for multi-electron atoms. Niel Bohrs Atomic Theory states that an atom is like a planetary model where electrons were situated in discretely energized orbits.

B concentrated in the center of an atom. An electron shifts from a lower energy level to a higher one. A Most of the space in an atom is empty b The total number of neutrons and protons is always equal in a neutral atom c The total number of electrons and protons in an atom is always equal d The total number f electrons in any energy level can be calculated by the formula 2n2. Bohrs model of the structure of an atom includes ideas from both classical and quantum physics asked Sep 24 2016 in Physics Space Science by Pinchtour introductory-physics. Option C It explained the odds of finding the position of an electron.
 Source: universetoday.com
Source: universetoday.com
Bohr atomic model and the models after that explain the properties of atomic electrons on the basis of certain allowed possible values. The energy of the electron in a given orbit is not fixed. What was Niels Bohrs explanation for the observation of atomic spectra. A Most of the space in an atom is empty b The total number of neutrons and protons is always equal in a neutral atom c The total number of electrons and protons in an atom is always equal d The total number f electrons in any energy level can be calculated by the formula 2n2. Electrons need specific amounts of energy to jump off an atom and be emitted.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The Bohrs atomic model has atom consisting of a small positively-charged nucleus orbited by negative-charged electrons. A located in the space outside the nucleus. Each orbit has a specific energy level. Energy is absorbed and then released to form an emission line. Bohrs model of the structure of an atom includes ideas from both classical and quantum physics asked Sep 24 2016 in Physics Space Science by Pinchtour introductory-physics.

Energy is absorbed and then released to form an emission line. Bohrs model had one significant drawback - they were only a 2-D diagram of the truth. What was Niels Bohrs explanation for the observation of atomic spectra. Bohr atomic model and the models after that explain the properties of atomic electrons on the basis of certain allowed possible values. Electrons can exist in any energy level.
 Source: toppr.com
Source: toppr.com
Kumar is producing the photoelectric effect by using red light. The Bohr model and all of its successors describe the properties of. Which one of the following statements is a consequence of this model. Asked Jun 26 2017 in Chemistry by LittleBuddha. Bohrs most important work was developing a model of the atom.
 Source: pinterest.com
Source: pinterest.com
The main problem with Bohrs model is that it works very well for atoms with only one electron like H or He but not at all for multi-electron atoms. The Bohrs atomic model has atom consisting of a small positively-charged nucleus orbited by negative-charged electrons. What was Niels Bohrs explanation for the observation of atomic spectra. According to Niels Bohrs atomic model what occurs when an atom absorbs radiated energy. Electrons can exist in any energy level.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The atom would radiate a photon when an excited electron would jump down from a higher orbit to a lower orbit. Bohrs model of the structure of an atom includes ideas from both classical and quantum physics asked Sep 24 2016 in Physics Space Science by Pinchtour introductory-physics. B concentrated in the center of an atom. Higher orbits have lower energies. According to Niels Bohrs atomic model what occurs when an atom absorbs radiated energy.
 Source: doubtnut.com
Source: doubtnut.com
Bohr atomic model and the models after that explain the properties of atomic electrons on the basis of certain allowed possible values. Bohr model description of the structure of atoms especially that of hydrogen proposed 1913 by the Danish physicist Niels BohrThe Bohr model of the atom a radical departure from earlier classical descriptions was the first that incorporated quantum theory and was the predecessor of wholly quantum-mechanical models. It contradicted the Heisenberg uncertainty principle. Bohrs model of the structure of an atom includes ideas from both classical and quantum physics asked Sep 24 2016 in Physics Space Science by Pinchtour introductory-physics. What was Niels Bohrs explanation for the observation of atomic spectra.
 Source: academia.edu
Source: academia.edu
The difference between the energies of those orbits would be equal to the energy of the photon. The Bohrs atomic model has atom consisting of a small positively-charged nucleus orbited by negative-charged electrons. The energy of the electron in a given orbit is not fixed. Which one of the following statement is not true. Bohr atomic model and the models after that explain the properties of atomic electrons on the basis of certain allowed possible values.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title which statement about niels bohrs atomic model is true by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





