Your Which of the following is the best description of the first law of thermodynamics images are available in this site. Which of the following is the best description of the first law of thermodynamics are a topic that is being searched for and liked by netizens today. You can Get the Which of the following is the best description of the first law of thermodynamics files here. Find and Download all royalty-free photos.
If you’re looking for which of the following is the best description of the first law of thermodynamics pictures information linked to the which of the following is the best description of the first law of thermodynamics topic, you have visit the right blog. Our website always provides you with hints for viewing the highest quality video and image content, please kindly surf and find more enlightening video articles and graphics that fit your interests.
Which Of The Following Is The Best Description Of The First Law Of Thermodynamics. Energy flow in diesel engine. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. Human metabolism is a complicated process. First Law of Thermodynamics.
 First Law Of Thermodynamics Equations Limitations Examples From byjus.com
First Law Of Thermodynamics Equations Limitations Examples From byjus.com
Previous question Next question. The first takes place in the north between the Union and the Northmen. The first law makes use of the key concepts of internal energy heat and system workIt is used extensively in the discussion of heat enginesThe standard unit for all these quantities would be the joule although they are sometimes. The change in the internal energy of a system is the sum of the heat transferred and the work done. Human metabolism is a complicated process. Our body provides a good example of irreversible processes.
The plot of the original trilogy involves three major powers.
Isaac Newton a 17th century scientist put forth a variety of laws that explain why objects move or dont move as they do. Energy can be changed from one form to another but it cannot be created or destroyed. The First Law of Thermodynamics is the Law of Conservation of Energy - in other words the total amount of energy in a closed system remains constant. Newtons first law of motion is often stated as. For example exercising changes energy from food into kinetic energy. The law forms the basis of the principle of conservation of energy.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The first law describes how energy cannot be created or destroyed merely transformed from one kind to another. TopExamples of the First Law of Thermodynamics also known as the Conservation of Energy Law. The first law asserts that if heat is recognized as a form of energy then the total energy of a system plus its surroundings is conserved. Energy flow in diesel engine. The first law describes how energy cannot be created or destroyed merely transformed from one kind to another.
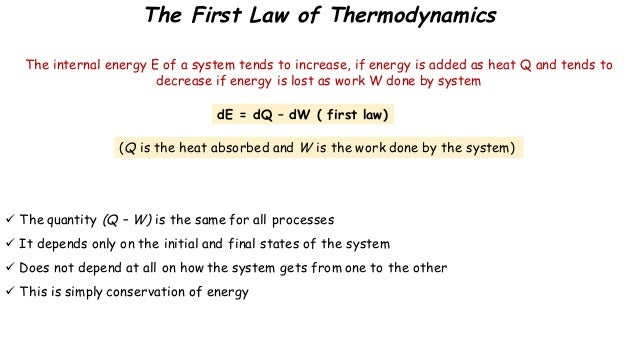 Source: slideshare.net
Source: slideshare.net
For example exercising changes energy from food into kinetic energy. In other words the total energy of the universe remains constant. The first law asserts that if heat is recognized as a form of energy then the total energy of a system plus its surroundings is conserved. The second equation is a way to express the second law of thermodynamics in. The laws of thermodynamics are deceptively simple to state but they are far-reaching in their consequences.
 Source: byjus.com
Source: byjus.com
The first law of thermodynamics states that energy can neither be created nor destroyed. Previous question Next question. There are three places this internal energy can goto heat transfer to doing work and to stored fat. First Law of Thermodynamics The first law of thermodynamics is the application of the conservation of energy principle to heat and thermodynamic processes. The first law of thermodynamics states that the energy of the universe is constant.

The First Law of Thermodynamics is the Law of Conservation of Energy - in other words the total amount of energy in a closed system remains constant. The first law asserts that if heat is recognized as a form of energy then the total energy of a system plus its surroundings is conserved. The 1st law of thermodynamics describes the beginning and ending points of these processes. Energy flow in diesel engine. The second equation is a way to express the second law of thermodynamics in.
 Source: lawofthermodynamicsinfo.com
Source: lawofthermodynamicsinfo.com
There are two major theaters of war. First Law of Thermodynamics The first law of thermodynamics is the application of the conservation of energy principle to heat and thermodynamic processes. Thermodynamics is the study of energy. Newtons first law of motion is often stated as. First Law of Thermodynamics.
 Source: pinterest.com
Source: pinterest.com
The first takes place in the north between the Union and the Northmen. The 1st law of thermodynamics describes the beginning and ending points of these processes. The first law describes how energy cannot be created or destroyed merely transformed from one kind to another. Energy flow in diesel engine. TopExamples of the First Law of Thermodynamics also known as the Conservation of Energy Law.
 Source: physicsabout.com
Source: physicsabout.com
TopExamples of the First Law of Thermodynamics also known as the Conservation of Energy Law. Energy can be changed from one form to another but it cannot be created or destroyed. Energy flow in diesel engine. The plot of the original trilogy involves three major powers. The First Law of Thermodynamics is the Law of Conservation of Energy - in other words the total amount of energy in a closed system remains constant.
 Source: slideshare.net
Source: slideshare.net
There are two major theaters of war. The second equation is a way to express the second law of thermodynamics in. The 1st law of thermodynamics describes the beginning and ending points of these processes. The first law of thermodynamics states that energy can neither be created nor destroyed. Previous question Next question.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The first law makes use of the key concepts of internal energy heat and system workIt is used extensively in the discussion of heat enginesThe standard unit for all these quantities would be the joule although they are sometimes. The law forms the basis of the principle of conservation of energy. First Law of Thermodynamics The first law of thermodynamics is the application of the conservation of energy principle to heat and thermodynamic processes. Thermodynamics is the study of energy. Isaac Newton a 17th century scientist put forth a variety of laws that explain why objects move or dont move as they do.
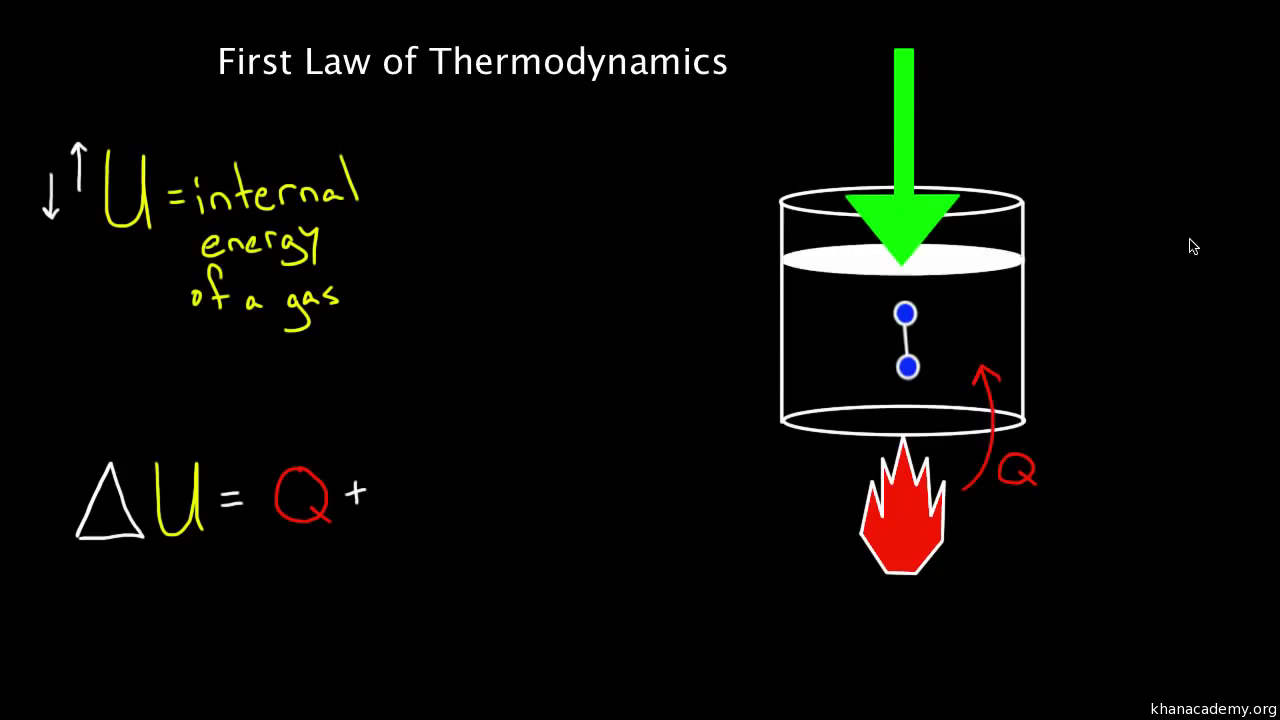 Source: khanacademy.org
Source: khanacademy.org
The first law of thermodynamics applies the conservation of energy principle to systems where heat transfer and doing work are the methods of transferring energy into and out of the system. Energy flow in diesel engine. The focus of Lesson 1 is Newtons first law of motion - sometimes referred to as the law of inertia. The change in the internal energy of a system is the sum of the heat transferred and the work done. The first law of thermodynamics applies the conservation of energy principle to systems where heat transfer and doing work are the methods of transferring energy into and out of the system.
 Source: byjus.com
Source: byjus.com
Newtons first law of motion is often stated as. The first law of thermodynamics states that energy can neither be created nor destroyed. First Law of Thermodynamics. Energy flow in diesel engine. The second equation is a way to express the second law of thermodynamics in.
 Source: lawofthermodynamicsinfo.com
Source: lawofthermodynamicsinfo.com
The change in the internal energy of a system is the sum of the heat transferred and the work done. Our body loses internal energy. Previous question Next question. The first law describes how energy cannot be created or destroyed merely transformed from one kind to another. First Law of Thermodynamics.
 Source: slideshare.net
Source: slideshare.net
Answer The change in internal energy of a system is equal to the energy transfered into or out of the system as workheat or both explanation The first law of thermodynamics is based on the Princi view the full answer. The 1st law of thermodynamics describes the beginning and ending points of these processes. The first law describes how energy cannot be created or destroyed merely transformed from one kind to another. There are two major theaters of war. Energy flow in diesel engine.
 Source: lawofthermodynamicsinfo.com
Source: lawofthermodynamicsinfo.com
The first law of thermodynamics states that the energy of the universe is constant. The change in the internal energy of a system is the sum of the heat transferred and the work done. Human metabolism is a complicated process. The first law of thermodynamics applies the conservation of energy principle to systems where heat transfer and doing work are the methods of transferring energy into and out of the system. Previous question Next question.
 Source: slideshare.net
Source: slideshare.net
First Law of Thermodynamics The first law of thermodynamics is the application of the conservation of energy principle to heat and thermodynamic processes. The first law of thermodynamics applies the conservation of energy principle to systems where heat transfer and doing work are the methods of transferring energy into and out of the system. Human metabolism is a complicated process. The Union the Gurkish Empire and the North recently united under King Bethod. First Law of Thermodynamics.
 Source: khanacademy.org
Source: khanacademy.org
The 1st law of thermodynamics describes the beginning and ending points of these processes. Answer The change in internal energy of a system is equal to the energy transfered into or out of the system as workheat or both explanation The first law of thermodynamics is based on the Princi view the full answer. The total amount of energy and matter in the Universe remains constant merely changing from one form to another. The first law of thermodynamics applies the conservation of energy principle to systems where heat transfer and doing work are the methods of transferring energy into and out of the system. For example exercising changes energy from food into kinetic energy.

Energy flow in diesel engine. Newtons first law of motion is often stated as. Our body loses internal energy. This means that if you know the rules you like engineers and scientists can often predict what will happen and what wont happen. The 1st law of thermodynamics describes the beginning and ending points of these processes.
 Source: lawofthermodynamicsinfo.com
Source: lawofthermodynamicsinfo.com
Human metabolism is a complicated process. The first takes place in the north between the Union and the Northmen. The First Law of Thermodynamics with Examples Our changeable friend energy with its multiple personalities is sometimes hard to keep track of and even harder to defineBut it does always behave in predictable ways. In other words the total energy of the universe remains constant. Thermodynamics - thermodynamics - The first law of thermodynamics.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title which of the following is the best description of the first law of thermodynamics by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





