Your The second law of thermodynamics states that _____ images are available. The second law of thermodynamics states that _____ are a topic that is being searched for and liked by netizens today. You can Download the The second law of thermodynamics states that _____ files here. Download all free photos and vectors.
If you’re searching for the second law of thermodynamics states that _____ pictures information linked to the the second law of thermodynamics states that _____ keyword, you have visit the right blog. Our website frequently provides you with hints for refferencing the highest quality video and image content, please kindly search and find more enlightening video articles and images that match your interests.
The Second Law Of Thermodynamics States That _____. Being a law of thermodynamics it deals with heat. The second law of thermodynamics states that the entropy of the universe increases during a spontaneous process. In simple words the law explains that an isolated systems entropy will never decrease over time. Due to entropy which is the measure of disorder in a closed system all of the available energy will not be useful to the organism.
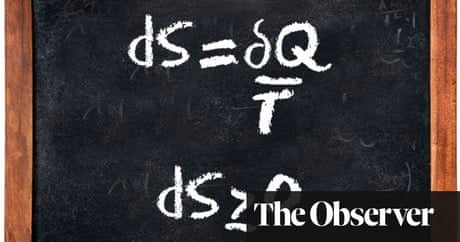 What Is The Second Law Of Thermodynamics Science The Guardian From theguardian.com
What Is The Second Law Of Thermodynamics Science The Guardian From theguardian.com
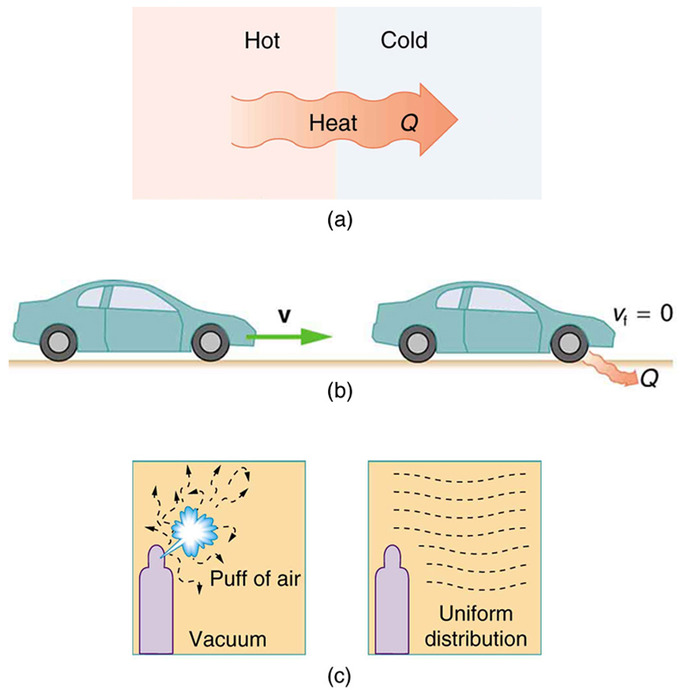
Entropy increases as energy is transferred. The law states that it is impossible for any process to have as its sole result heat transfer from a cooler to a hotter. The Second Law of Thermodynamics is about the quality of energy. The second law of thermodynamics is also the Law of Entropy. The second law of thermodynamics states. Delta S delta Q T For a given physical process the combined entropy of the system and the environment remains a constant if the process can be reversed.
The second law states that there exists a useful state variable called entropy S.
The third law of thermodynamics establishes the zero for entropy as that of a perfect pure crystalline solid at 0 K. The second law of thermodynamics states. As we know that entropy is the degree of disorder present in a substance. The second law of thermodynamics states that in a natural thermodynamic process the sum of the entropies of the interacting thermodynamic systems never decreases. The second law of thermodynamics states. An important implication of this law is that heat transfers energy spontaneously from higher- to lower-temperature objects but never spontaneously in the reverse direction.
 Source: chegg.com
Source: chegg.com
Being a law of thermodynamics it deals with heat. The second law of thermodynamics states that the world as a whole is always in a state of positive entropy. Another form of the statement is that heat does not spontaneously pass from a colder body to a warmer body. If Δ Suniv 0 the process is nonspontaneous and if Δ Suniv 0 the system is at equilibrium. Heat transfer occurs spontaneously from higher- to lower-temperature bodies but never spontaneously in the reverse direction.
The second equation is a way to express the second law of thermodynamics in terms of entropy. The third law of thermodynamics establishes the zero for entropy as that of a perfect pure crystalline solid at 0 K. The second law of thermodynamics states. The first law of thermodynamics asserts that energy must be conserved in any process involving the exchange of heat and work between a system and its surroundings. Every system left to its own devices always tends to move from order to disorder its energy tending to be transformed into lower levels of availability for work ultimately becoming totally random and unavailable for work.
 Source: sciencedirect.com
Source: sciencedirect.com
According to the New Unity Physics the universe is defined as a living being that on Earth resembles an angiosperm plant. A machine that violated the first law would be called a perpetual motion machine of the first kind because it would manufacture its own energy out of nothing and thereby run forever. Delta S delta Q T For a given physical process the combined entropy of the system and the environment remains a constant if the process can be reversed. The second law of thermodynamics is a physical law that is not symmetric to reversal of the time direction. According to the New Unity Physics the universe is defined as a living being that on Earth resembles an angiosperm plant.
 Source: livescience.com
Source: livescience.com
Contributors and Attributions. An important implication of this law is that heat transfers energy spontaneously from higher- to lower-temperature objects but never spontaneously in the reverse direction. According to the New Unity Physics the universe is defined as a living being that on Earth resembles an angiosperm plant. Delta S delta Q T For a given physical process the combined entropy of the system and the environment remains a constant if the process can be reversed. A machine that violated the first law would be called a perpetual motion machine of the first kind because it would manufacture its own energy out of nothing and thereby run forever.
 Source: learn.lif.co.id
Source: learn.lif.co.id
The second law of thermodynamics does allow local decreases of entropy so long as the entropy of the entire isolated system increases. It states that as energy is transferred or transformed more and more of it is wasted. The Second Law also states that there is a. The Second Law of Thermodynamics is about the quality of energy. The second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous process.
 Source: sanfoundry.com
Source: sanfoundry.com
The second law of thermodynamics states that a spontaneous process increases the entropy of the universe Suniv 0. The second law also states that the changes in the entropy in the universe can never be negative. The second law states that there exists a useful state variable called entropy S. Another form of the statement is that heat does not spontaneously pass from a colder body to a warmer body. The second law of thermodynamics states.
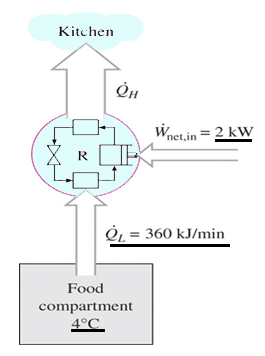 Source: sanfoundry.com
Source: sanfoundry.com
The second law of thermodynamics states that in a natural thermodynamic process the sum of the entropies of the interacting thermodynamic systems never decreases. This does not conflict with symmetries observed in the fundamental laws of physics particularly CPT symmetry since the second law applies statistically on. Another form of the statement is that heat does not spontaneously pass from a colder body to a warmer body. Positive entropy HOWEVER NOT AROUND A BLACK HOLE Q-FFF Theory. Being a law of thermodynamics it deals with heat.
 Source: sciencedirect.com
Source: sciencedirect.com
In simple words the law explains that an isolated systems entropy will never decrease over time. In simple words the law explains that an isolated systems entropy will never decrease over time. The second law of thermodynamics states that in a natural thermodynamic process the sum of the entropies of the interacting thermodynamic systems never decreases. The Second Law also states that there is a. Being a law of thermodynamics it deals with heat.
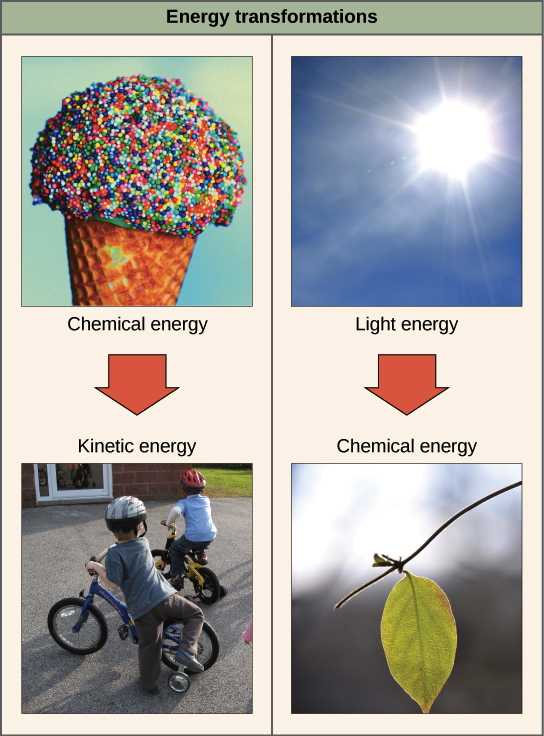 Source: khanacademy.org
Source: khanacademy.org
The second law of thermodynamics states. Another form of the statement is that heat does not spontaneously pass from a colder body to a warmer body. Due to entropy which is the measure of disorder in a closed system all of the available energy will not be useful to the organism. The third law of thermodynamics establishes the zero for entropy as that of a perfect pure crystalline solid at 0 K. The Second Law also states that there is a.
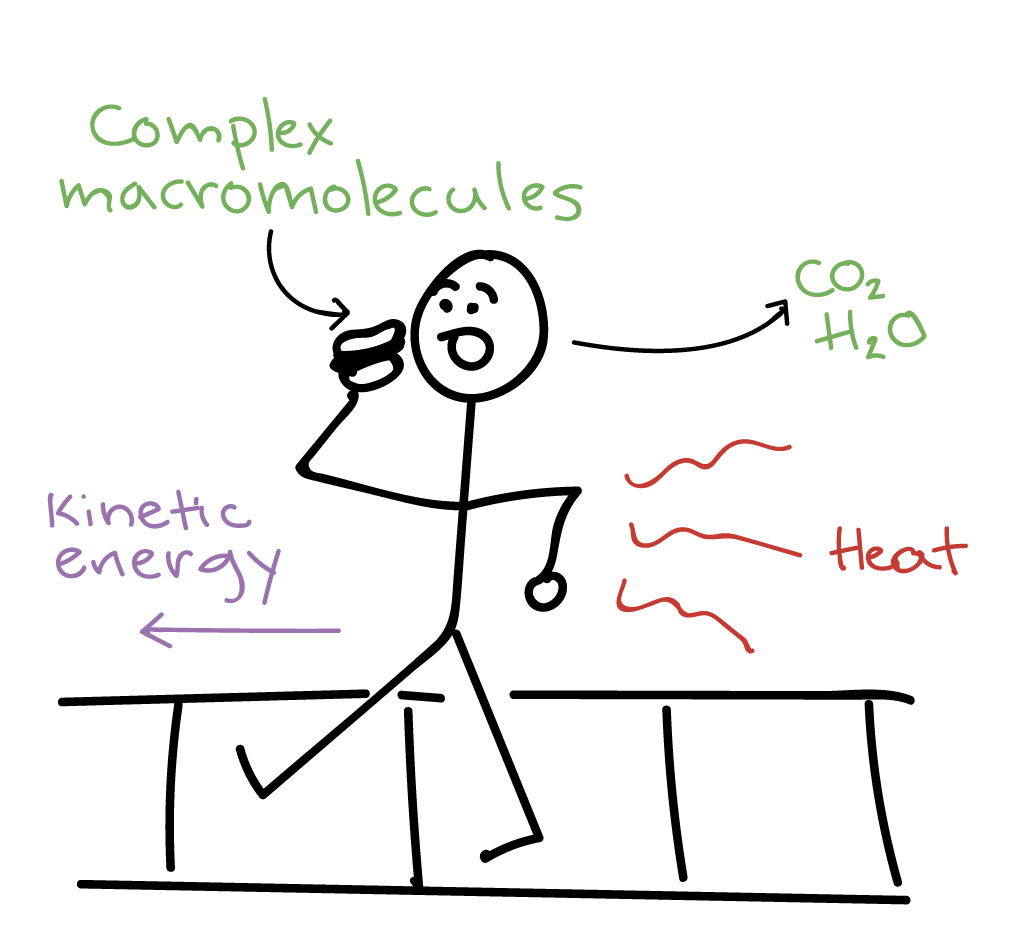 Source: khanacademy.org
Source: khanacademy.org
The second law of thermodynamics states that a spontaneous process increases the entropy of the universe Suniv 0. Due to entropy which is the measure of disorder in a closed system all of the available energy will not be useful to the organism. A machine that violated the first law would be called a perpetual motion machine of the first kind because it would manufacture its own energy out of nothing and thereby run forever. The second law of thermodynamics states that a spontaneous process increases the entropy of the universe Suniv 0. The first law of thermodynamics asserts that energy must be conserved in any process involving the exchange of heat and work between a system and its surroundings.
 Source: pinterest.com
Source: pinterest.com
The second law states that there exists a useful state variable called entropy S. The formula says that the entropy of an isolated. An important implication of this law is that heat transfers energy spontaneously from higher- to lower-temperature objects but never spontaneously in the reverse direction. The Second Law is a Thermodynamic Law We already have enough information to refute some of the myths surrounding the second law of thermodynamics. As we know that entropy is the degree of disorder present in a substance.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The Second Law also states that there is a. According to which the entropy of a closed system that is not in equilibrium increases over time reaching its maximum value when equilibrium occurs. The change in entropy delta S is equal to the heat transfer delta Q divided by the temperature T. The second law also states that the changes in the entropy in the universe can never be negative. Heat transfer occurs spontaneously from higher- to lower-temperature bodies but never spontaneously in the reverse direction.
 Source: pinterest.com
Source: pinterest.com
The second law of thermodynamics states that any spontaneously occurring process will always lead to an escalation in the entropy S of the universe. The law states that it is impossible for any process to have as its sole result heat transfer from a cooler to a hotter. The Second Law also states that there is a. The second law of thermodynamics states. Being a law of thermodynamics it deals with heat.
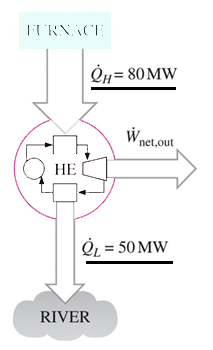 Source: sanfoundry.com
Source: sanfoundry.com
A machine that violated the first law would be called a perpetual motion machine of the first kind because it would manufacture its own energy out of nothing and thereby run forever. Delta S delta Q T For a given physical process the combined entropy of the system and the environment remains a constant if the process can be reversed. The first law of thermodynamics asserts that energy must be conserved in any process involving the exchange of heat and work between a system and its surroundings. The second law of thermodynamics is a physical law that is not symmetric to reversal of the time direction. The second equation is a way to express the second law of thermodynamics in terms of entropy.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The second law of thermodynamics. The second equation is a way to express the second law of thermodynamics in terms of entropy. The law states that it is impossible for any process to have as its sole result heat transfer from a cooler to a hotter. The third law of thermodynamics establishes the zero for entropy as that of a perfect pure crystalline solid at 0 K. The Second Law is a Thermodynamic Law We already have enough information to refute some of the myths surrounding the second law of thermodynamics.
 Source: slideplayer.com
Source: slideplayer.com
The Second Law is a Thermodynamic Law We already have enough information to refute some of the myths surrounding the second law of thermodynamics. It states that as energy is transferred or transformed more and more of it is wasted. The law states that it is impossible for any process to have as its sole result heat transfer from a cooler to a hotter. This does not conflict with symmetries observed in the fundamental laws of physics particularly CPT symmetry since the second law applies statistically on. Positive entropy HOWEVER NOT AROUND A BLACK HOLE Q-FFF Theory.
 Source: pinterest.com
Source: pinterest.com
HOWEVER NOT AROUND A BLACK HOLE at the trapped horizonsee Q-FFF Theory below together with Penrose trapped space proposal. The second law of thermodynamics states that a spontaneous process increases the entropy of the universe Suniv 0. The second law of thermodynamics states that any spontaneously occurring process will always lead to an escalation in the entropy S of the universe. It states that as energy is transferred or transformed more and more of it is wasted. The formula says that the entropy of an isolated.
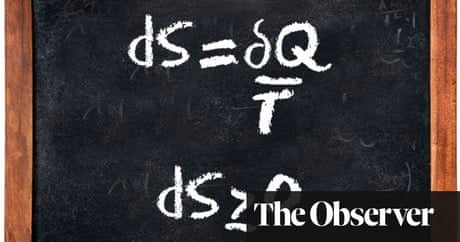 Source: theguardian.com
Source: theguardian.com
HOWEVER NOT AROUND A BLACK HOLE at the trapped horizonsee Q-FFF Theory below together with Penrose trapped space proposal. The first law of thermodynamics asserts that energy must be conserved in any process involving the exchange of heat and work between a system and its surroundings. The second law of thermodynamics states that the entropy of the universe increases during a spontaneous process. The second law of thermodynamics states. The law states that it is impossible for any process to have as its sole result heat transfer from a cooler to a hotter.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title the second law of thermodynamics states that _____ by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





