Your Potassium freezing point images are ready in this website. Potassium freezing point are a topic that is being searched for and liked by netizens now. You can Get the Potassium freezing point files here. Get all royalty-free photos.
If you’re looking for potassium freezing point pictures information connected with to the potassium freezing point interest, you have visit the right blog. Our site always gives you suggestions for seeing the highest quality video and picture content, please kindly surf and locate more informative video articles and graphics that match your interests.
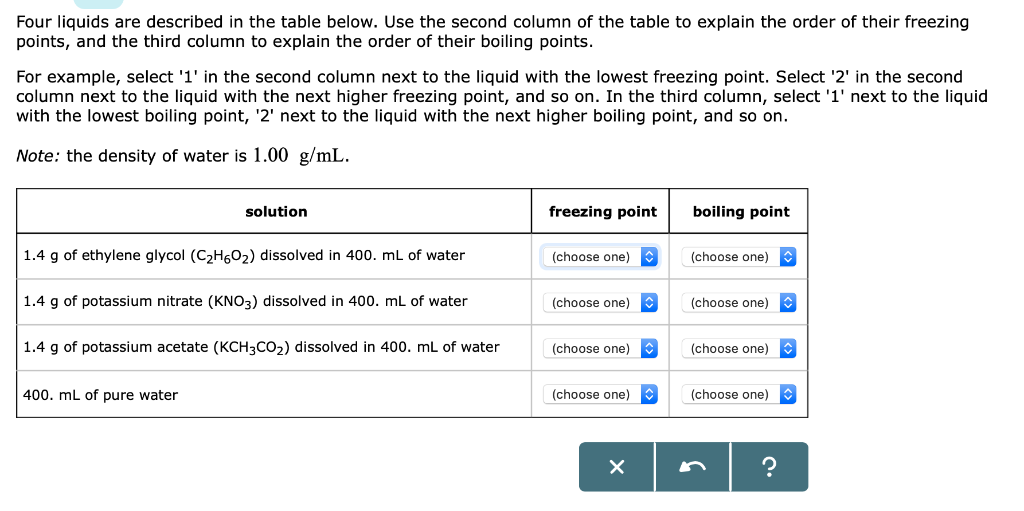
Potassium Freezing Point. Key value for chemical safety assessment Melting freezing point at 101 325 Pa. One chart is for Fahrenheit and the other is for Centigrade. Boiling Point FreezingMelting Point. M Molality moles of solute per kg of water.
 Freezing Point Depression Ck 12 Foundation From ck12.org
Freezing Point Depression Ck 12 Foundation From ck12.org
SPECIFIC GRAVITY 145 PARTITION COEFFICIENT. In general melting is a phase change of a substance from the solid to the liquid phase. Potassium Melting Point. Boiling point of saturated solution at 1013 bar 1086 C. Note that CaCl 2 is substantially more effective at lowering the freezing point of water because its solutions contain three ions per formula unit. Thus the use of potassium perchlorate has been extensive for hyperthyroidism during the.
-5 to 62 F -21 to 17 C.
Boiling Point Saturation. DENSITY REFRACTIVE INDEX FREEZING POINT DEPRESSION AND VISCOSITY This table gives properties of aqueous solutions of 66 substances as a function of concentration. Boiling point of saturated solution at 1013 bar 1086 C. Potassium Melting Point. The freezing point of aqueous potassium nitrate is 20 C 20 C. You would use 200 grams of KOH with 800 ml of water.
 Source: researchgate.net
Source: researchgate.net
Mass of solute divided by total mass of solution expressed as percent. It lowers the freezing point from zero Celsius 34 Fahrenheit to about two Celsius salt used on roads will lower the freezing point more than sea salt which is what I used. Boiling point C 146 Freezing point C 10 Viscosity at 20 kgms 610-3 CHEMICAL PROPERTIES Potassium hydroxide solution 50 produces an alkaline reaction that attacks certain metals including aluminum copper and their alloys. Potassium formate functions by depressing the freezing point of water to melt ice and snow accumulation and prevent re-freezing. Thus the use of potassium perchlorate has been extensive for hyperthyroidism during the.

CRC Handbook of Chemistry and Physics 85th Edition 2003 reports a melting point of 525 C for potassium perchlorate. Heat of fusion 3377 kJmol. Boiling point of Potassium is 760C. Potassium Melting Point. The freezing point of aqueous potassium nitrate is 20 C 20 C.
 Source: chegg.com
Source: chegg.com
-5 to 62 F -21 to 17 C. Note that these points are associated with the standard atmospheric pressure. SPECIFICATIONS SAFETY AND HANDLING INFORMATION. Freezing point depression constant for water is 186 C. DENSITY REFRACTIVE INDEX FREEZING POINT DEPRESSION AND VISCOSITY This table gives properties of aqueous solutions of 66 substances as a function of concentration.
 Source: doubtnut.com
Source: doubtnut.com
SPECIFIC GRAVITY 145 PARTITION COEFFICIENT. Potassium perchlorate acts as a competitive inhibitor of iodine uptake by the thyroid gland and attenuates the production of the thyroid hormone. The freezing point of aqueous potassium nitrate is 20 C 20 C. Boiling point of saturated solution at 1013 bar 1086 C. SPECIFIC GRAVITY 145 PARTITION COEFFICIENT.
 Source: researchgate.net
Source: researchgate.net
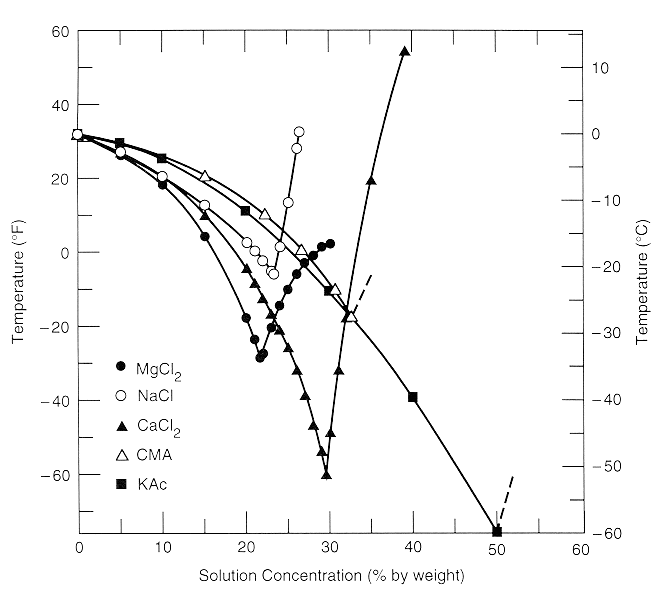
The temperatures at which various KOH concentrations will freeze can be found in the charts below. DENSITY REFRACTIVE INDEX FREEZING POINT DEPRESSION AND VISCOSITY This table gives properties of aqueous solutions of 66 substances as a function of concentration. Melting point of Potassium is 6325C. Entropy S 8255 Jmol-K. Note that CaCl 2 is substantially more effective at lowering the freezing point of water because its solutions contain three ions per formula unit.
 Source: sciencedirect.com
Source: sciencedirect.com
SPECIFIC GRAVITY 145 PARTITION COEFFICIENT. Melting point of Potassium is 6325C. MELTING POINTFREEZING POINT -20 F -2889 C FLAMMABILITY Not Applicable PERCENT VOLATILE 55 estimated UPPERLOWER FLAMMABILITY EXPLOSIVE LIMITS Not Applicable RELATIVE DENSITY Not Available DENSITY 1210 lbsgal. -5 to 62 F -21 to 17 C. 1 ml of water 1 gram.
 Source: slideplayer.com
Source: slideplayer.com
Note that CaCl 2 is substantially more effective at lowering the freezing point of water because its solutions contain three ions per formula unit. Freezing point depression constant for water is 186 C. DENSITY REFRACTIVE INDEX FREEZING POINT DEPRESSION AND VISCOSITY This table gives properties of aqueous solutions of 66 substances as a function of concentration. Not applicable to liquids. SPECIFIC GRAVITY 145 PARTITION COEFFICIENT.
 Source: fhwa.dot.gov
Source: fhwa.dot.gov
MELTING POINTFREEZING POINT -20 F -2889 C FLAMMABILITY Not Applicable PERCENT VOLATILE 55 estimated UPPERLOWER FLAMMABILITY EXPLOSIVE LIMITS Not Applicable RELATIVE DENSITY Not Available DENSITY 1210 lbsgal. Heat of fusion 3377 kJmol. All data refer to a temperature of 20C. Because the freezing point of pure water is 0C the actual freezing points of the solutions are 22C and 30C respectively. Entropy S 8255 Jmol-K.
 Source: ck12.org
Source: ck12.org
The solute of the solution is potassium sulfate K2SO4 K 2 S O 4 while its solvent is water. The temperatures at which various KOH concentrations will freeze can be found in the charts below. Thermal coeff of expansion 15-25 C 337X10-6K. Freezing point of Potassium K is 6338 C Convert 6338 C to different units. It lowers the freezing point from zero Celsius 34 Fahrenheit to about two Celsius salt used on roads will lower the freezing point more than sea salt which is what I used.
 Source: fuelsaver-mpg.com
Source: fuelsaver-mpg.com
The freezing point of aqueous potassium nitrate is 20 C 20 C. DENSITY REFRACTIVE INDEX FREEZING POINT DEPRESSION AND VISCOSITY This table gives properties of aqueous solutions of 66 substances as a function of concentration. Mass of solute divided by total mass of solution expressed as percent. One chart is for Fahrenheit and the other is for Centigrade. Once it is effectively applied to a runway surface the solution becomes diluted with a practical freezing point of -32C -26F at 50 concentration.
 Source: socratic.org
Source: socratic.org
SPECIFICATIONS SAFETY AND HANDLING INFORMATION. The mass of water is 05 kg. MELTING POINTFREEZING POINT -20 F -2889 C FLAMMABILITY Not Applicable PERCENT VOLATILE 55 estimated UPPERLOWER FLAMMABILITY EXPLOSIVE LIMITS Not Applicable RELATIVE DENSITY Not Available DENSITY 1210 lbsgal. This would make a 20 solution of KOH by. It lowers the freezing point from zero Celsius 34 Fahrenheit to about two Celsius salt used on roads will lower the freezing point more than sea salt which is what I used.
 Source: slideplayer.com
Source: slideplayer.com
Boiling point of Potassium is 760C. Boiling point C 146 Freezing point C 10 Viscosity at 20 kgms 610-3 CHEMICAL PROPERTIES Potassium hydroxide solution 50 produces an alkaline reaction that attacks certain metals including aluminum copper and their alloys. Melting point of Potassium is 6325C. MELTING POINTFREEZING POINT -20 F -2889 C FLAMMABILITY Not Applicable PERCENT VOLATILE 55 estimated UPPERLOWER FLAMMABILITY EXPLOSIVE LIMITS Not Applicable RELATIVE DENSITY Not Available DENSITY 1210 lbsgal. Not applicable to liquids.
 Source: researchgate.net
Source: researchgate.net
Thermal coeff of expansion 15-25 C 337X10-6K. 221-241 F 105-116 C Freezing PointRange. Melting point of Potassium is 6325C. It lowers the freezing point from zero Celsius 34 Fahrenheit to about two Celsius salt used on roads will lower the freezing point more than sea salt which is what I used. Potassium formate functions by depressing the freezing point of water to melt ice and snow accumulation and prevent re-freezing.

Melting point of Potassium is 6325C. Boiling point C 146 Freezing point C 10 Viscosity at 20 kgms 610-3 CHEMICAL PROPERTIES Potassium hydroxide solution 50 produces an alkaline reaction that attacks certain metals including aluminum copper and their alloys. Boiling Point Saturation. Not applicable to liquids. CRC Handbook of Chemistry and Physics 85th Edition 2003 reports a melting point of 525 C for potassium perchlorate.
 Source: chegg.com
Source: chegg.com
Boiling point C 146 Freezing point C 10 Viscosity at 20 kgms 610-3 CHEMICAL PROPERTIES Potassium hydroxide solution 50 produces an alkaline reaction that attacks certain metals including aluminum copper and their alloys. Boiling Point Saturation. Note that these points are associated with the standard atmospheric pressure. The temperatures at which various KOH concentrations will freeze can be found in the charts below. Once it is effectively applied to a runway surface the solution becomes diluted with a practical freezing point of -32C -26F at 50 concentration.

The freezing point of aqueous potassium nitrate is 20 C 20 C. Boiling point C 146 Freezing point C 10 Viscosity at 20 kgms 610-3 CHEMICAL PROPERTIES Potassium hydroxide solution 50 produces an alkaline reaction that attacks certain metals including aluminum copper and their alloys. It lowers the freezing point from zero Celsius 34 Fahrenheit to about two Celsius salt used on roads will lower the freezing point more than sea salt which is what I used. Not applicable to liquids. Enthalpy of formation -4367 kJmol.
 Source: slideplayer.com
Source: slideplayer.com
SPECIFIC GRAVITY 145 PARTITION COEFFICIENT. SPECIFIC GRAVITY 145 PARTITION COEFFICIENT. Thus the use of potassium perchlorate has been extensive for hyperthyroidism during the. One chart is for Fahrenheit and the other is for Centigrade. Heat of fusion 3377 kJmol.
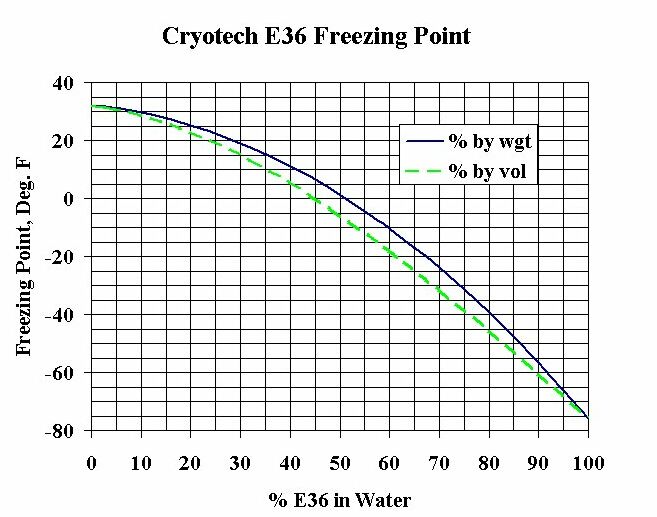 Source: p2infohouse.org
Source: p2infohouse.org
Thus the use of potassium perchlorate has been extensive for hyperthyroidism during the. Melting point of Potassium is 6325C. CRC Handbook of Chemistry and Physics 85th Edition 2003 reports a melting point of 525 C for potassium perchlorate. This only doesnt work if the temperature of the air is much lower below 15 Fahrenheit I believe than freezing point. Boiling point of saturated solution at 1013 bar 1086 C.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title potassium freezing point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





