Your Hydrogen peroxide chlorine reaction images are ready. Hydrogen peroxide chlorine reaction are a topic that is being searched for and liked by netizens today. You can Download the Hydrogen peroxide chlorine reaction files here. Get all free photos.
If you’re searching for hydrogen peroxide chlorine reaction pictures information linked to the hydrogen peroxide chlorine reaction keyword, you have pay a visit to the right site. Our site always gives you hints for seeking the highest quality video and image content, please kindly hunt and find more informative video content and graphics that fit your interests.
Hydrogen Peroxide Chlorine Reaction. ClO H 2 O 2 Cl H 2 O O 2. Bubbling chlorine gas through hydrogen peroxide. Hydrogen perioxide is oxidized to oxxygen by strong oxidizing agents such as acidic KMnO 4 or K 2 CrO 4 or K Cr 2 O 7. The hydrochloric acid thus formed would attack the remaining chlorate the products of the reaction being chlorine and chlorine dioxide the chlorine then reacting with more hydrogen peroxide to again form hydrogen chloride.
 Pdf Decomposition Of Hydrogen Peroxide Kinetics And Review Of Chosen Catalysts From researchgate.net
Pdf Decomposition Of Hydrogen Peroxide Kinetics And Review Of Chosen Catalysts From researchgate.net
Hydrogen perioxide is oxidized to oxxygen by strong oxidizing agents such as acidic KMnO 4 or K 2 CrO 4 or K Cr 2 O 7. While there is no upper limit to the pH eg H2O2 can be used to dechlorinate effluent from causticchlorine odor scrubbers as a practical matter pH 85 is preferred in order to provide an instantaneous reaction. Without chlorine or active hydrogen peroxide acting as a sanitizer bacteria and other contaminants can pollute a pool in short order. IV administration of hydrogen peroxide can result in inflammation at the injection site gas embolism and life-threatening allergic reactions. The reaction between solutions of chloric acid HClO3 and hydrogen peroxide does not have any appreciable reaction rate until a temperatures above 70degC. Metal salts reaction with hydrogen peroxide.
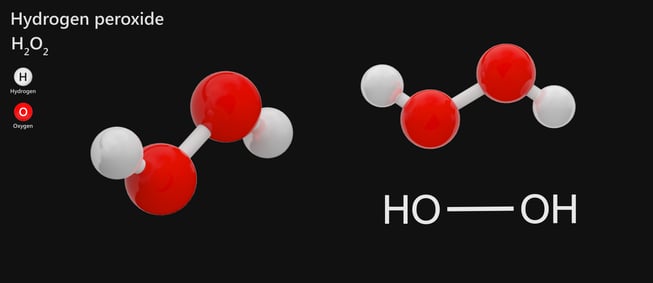
Hydrogen peroxide is a chemical compound with the formula H 2 O 2In its pure form it is a very pale blue liquid slightly more viscous than waterIt is used as an oxidizer bleaching agent and antisepticConcentrated hydrogen peroxide or high-test peroxide is a reactive oxygen species and has been used as a propellant in rocketryIts chemistry is dominated by the OO bond.
But in my own objective opinion I would personally choose hydrogen peroxide primarily because it does not leave any residual. The reaction is started by shining a small ultraviolet LED onto the test tube. Hydrogen peroxide 35 is not approved by the FDA for any purpose. These metrics are regularly updated to reflect usage leading up to the last few days. But in my own objective opinion I would personally choose hydrogen peroxide primarily because it does not leave any residual. For certain purposes potable water needs to be freed of its residual chlorine.
 Source: youtube.com
Source: youtube.com
Hydrogen and Chlorine react explosively to form Hydrogen Chloride. Kinetics of hydrogen peroxide-chlorate reaction in the formation of chlorine dioxide. The two agents react quantitatively and stoichiometrically according to. Chem50 465 year 1904. Silver oxide is reduced to silver by hydrogen peroxide.
 Source: sciencedirect.com
Source: sciencedirect.com
In this process there is a side-reaction in which some chlorine dioxide is consumed by reaction with hydrogen peroxide. Hydrogen and Chlorine react explosively to form Hydrogen Chloride. Hydrogen peroxide is a chemical compound with the formula H 2 O 2In its pure form it is a very pale blue liquid slightly more viscous than waterIt is used as an oxidizer bleaching agent and antisepticConcentrated hydrogen peroxide or high-test peroxide is a reactive oxygen species and has been used as a propellant in rocketryIts chemistry is dominated by the OO bond. Hydrogen perioxide is oxidized to oxxygen by strong oxidizing agents such as acidic KMnO 4 or K 2 CrO 4 or K Cr 2 O 7. Kinetics of hydrogen peroxide-chlorate reaction in the formation of chlorine dioxide.
 Source: researchgate.net
Source: researchgate.net
So hydrogen peroxide can react with many compounds. But in my own objective opinion I would personally choose hydrogen peroxide primarily because it does not leave any residual. Hydrogen peroxide and chlorine dioxide are almost totally equal in mold remediation effectiveness. If hydrogen peroxide were poured into a pool it would quickly react with the chlorine-containing liquid bleach NaOCl in that pool and disable. Yet due to the prevalent use of liquid chlorine dioxide and its corrosive properties a stigma exists in which chlorine dioxide gas is also considered corrosive.
 Source: onlinelibrary.wiley.com
Source: onlinelibrary.wiley.com
Ferric chloride is reduced to ferrous cations by hydrogen peroxide. For certain purposes potable water needs to be freed of its residual chlorine. The reaction of chlorine dioxide with hydrogen peroxide was studied in a well stirred batch reactor in a pH range of 360 to 507 which is of interest for commercial chlorine dioxide bleaching of chemical pulp. Kinetics of hydrogen peroxide-chlorate reaction in the formation of chlorine dioxide. Bubbling chlorine gas through hydrogen peroxide.
 Source: researchgate.net
Source: researchgate.net
As shown in the table above chlorine dioxide has a relatively low oxidation corrosion potential 19 times lower than hydrogen peroxide. Ingestion of such preparations can cause serious harm or death. The reaction is completed within 1015 min. Oral ingestion of hydrogen peroxide can result in GI irritation and ulceration. Download Hi-Res Image Download to MS-PowerPoint.
 Source: researchgate.net
Source: researchgate.net
Hydrogen peroxide is highly oxidizing in acidic solutions outranking halogens and halogen compounds such as fluorine and chlorine dioxide in oxidation potential. Hydrogen peroxide 35 is not approved by the FDA for any purpose. Hydrogen peroxide oxidation by chlorine compounds. Fe 2 ions are oxidized to Fe 3 ions. If you have a bruised neck have unpleasant sensations in your muscles take a handkerchief and moisten it with hydrogen peroxide apply it to your neck and put a towel on top.
 Source: pinterest.com
Source: pinterest.com
The initial response from the Rio event staff was algae which is starting to sound more plausible now. It warms the muscles well and relieves pain. Initial rates of this side-reaction were measured in. So hydrogen peroxide can react with many compounds. In this process there is a side-reaction in which some chlorine dioxide is consumed by reaction with hydrogen peroxide.
 Source: blog.storemasta.com.au
Source: blog.storemasta.com.au
Ferric chloride is reduced to ferrous cations by hydrogen peroxide. Reaction dynamics and singlet oxygen formation. Hydrogen peroxide can act as an oxidizing or reducing agent at different pH values enabling its reaction with both metals and nonmetals such as iron and fluorine respectively. Initial rates of this side-reaction were measured in. Kinetics of hydrogen peroxide-chlorate reaction in the formation of chlorine dioxide.
 Source: researchgate.net
Source: researchgate.net
The initial response from the Rio event staff was algae which is starting to sound more plausible now. Algae probably could grow in the pool. Fe 2 ions are oxidized to Fe 3 ions. Oral ingestion of hydrogen peroxide can result in GI irritation and ulceration. Initial rates of this side-reaction were measured in.
 Source: pinterest.com
Source: pinterest.com
As means of dechlorination the reaction between hypochlorite and hydrogen peroxide was investigated. It warms the muscles well and relieves pain. IV administration of hydrogen peroxide can result in inflammation at the injection site gas embolism and life-threatening allergic reactions. Ferric chloride is reduced to ferrous cations by hydrogen peroxide. Silver oxide is reduced to silver by hydrogen peroxide.
 Source: pinterest.com
Source: pinterest.com
Yet due to the prevalent use of liquid chlorine dioxide and its corrosive properties a stigma exists in which chlorine dioxide gas is also considered corrosive. Chlorine is known to react with hydrogen peroxide to form hydrochloric acid and oxygen gas. Experiments conducted by Sand published in Zelt phys. IV administration of hydrogen peroxide can result in inflammation at the injection site gas embolism and life-threatening allergic reactions. Article Views are the COUNTER-compliant sum of full text article downloads since November 2008 both PDF and HTML across all institutions and individuals.
 Source: in.pinterest.com
Source: in.pinterest.com
The hydrochloric acid thus formed would attack the remaining chlorate the products of the reaction being chlorine and chlorine dioxide the chlorine then reacting with more hydrogen peroxide to again form hydrogen chloride. The reaction between solutions of chloric acid HClO3 and hydrogen peroxide does not have any appreciable reaction rate until a temperatures above 70degC. As shown in the table above chlorine dioxide has a relatively low oxidation corrosion potential 19 times lower than hydrogen peroxide. Experiments conducted by Sand published in Zelt phys. Fe 2 ions are oxidized to Fe 3 ions.
 Source: researchgate.net
Source: researchgate.net
Reaction dynamics and singlet oxygen formation. Hydrogen peroxide neutralizes chlorine. Silver oxide is reduced to silver by hydrogen peroxide. Article Views are the COUNTER-compliant sum of full text article downloads since November 2008 both PDF and HTML across all institutions and individuals. Experiments conducted by Sand published in Zelt phys.
 Source: youtube.com
Source: youtube.com
ClO H 2 O 2 Cl H 2 O O 2. Download Hi-Res Image Download to MS-PowerPoint. Kinetics of hydrogen peroxide-chlorate reaction in the formation of chlorine dioxide. If hydrogen peroxide were poured into a pool it would quickly react with the chlorine-containing liquid bleach NaOCl in that pool and disable. As shown in the table above chlorine dioxide has a relatively low oxidation corrosion potential 19 times lower than hydrogen peroxide.
 Source: classnotes.org.in
Source: classnotes.org.in
Based upon your hypothesis the peroxide will eject its oxygens and the products will be oxygen gas and hydrochloric acid. Initial rates of this side-reaction were measured in. Algae probably could grow in the pool. ClO H 2 O 2 Cl H 2 O O 2. The hydrochloric acid thus formed would attack the remaining chlorate the products of the reaction being chlorine and chlorine dioxide the chlorine then reacting with more hydrogen peroxide to again form hydrogen chloride.
 Source: pinterest.com
Source: pinterest.com
Based upon your hypothesis the peroxide will eject its oxygens and the products will be oxygen gas and hydrochloric acid. If you have a bruised neck have unpleasant sensations in your muscles take a handkerchief and moisten it with hydrogen peroxide apply it to your neck and put a towel on top. The reaction of chlorine dioxide with hydrogen peroxide was studied in a well stirred batch reactor in a pH range of 360 to 507 which is of interest for commercial chlorine dioxide bleaching of chemical pulp. Oral ingestion of hydrogen peroxide can result in GI irritation and ulceration. The two agents react quantitatively and stoichiometrically according to.
 Source: pinterest.com
Source: pinterest.com
Experiments conducted by Sand published in Zelt phys. The two agents react quantitatively and stoichiometrically according to. Hydrogen peroxide neutralizes chlorine. In this process there is a side-reaction in which some chlorine dioxide is consumed by reaction with hydrogen peroxide. While there is no upper limit to the pH eg H2O2 can be used to dechlorinate effluent from causticchlorine odor scrubbers as a practical matter pH 85 is preferred in order to provide an instantaneous reaction.
 Source: semanticscholar.org
Source: semanticscholar.org
Hydrogen peroxide reacts with free available chlorine in solutions with pH 7. The reaction is completed within 1015 min. Leave this compress for 10-20 minutes. Hydrogen peroxide can act as an oxidizing or reducing agent at different pH values enabling its reaction with both metals and nonmetals such as iron and fluorine respectively. Without chlorine or active hydrogen peroxide acting as a sanitizer bacteria and other contaminants can pollute a pool in short order.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title hydrogen peroxide chlorine reaction by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





