Your Does silicon conduct electricity images are available. Does silicon conduct electricity are a topic that is being searched for and liked by netizens today. You can Get the Does silicon conduct electricity files here. Find and Download all royalty-free photos and vectors.
If you’re searching for does silicon conduct electricity images information related to the does silicon conduct electricity interest, you have pay a visit to the ideal blog. Our site frequently gives you suggestions for seeing the maximum quality video and image content, please kindly search and locate more enlightening video articles and graphics that match your interests.
Does Silicon Conduct Electricity. Even though N-type silicon by itself is a conductor and P-type silicon by itself is also a conductor the combination shown in the diagram does not conduct any electricity. It is a hard brittle crystalline solid with a blue-grey metallic lustre and is a tetravalent metalloid and semiconductorIt is a member of group 14 in the periodic table. The resistivity data for silicon is widely available. Variable electrical conductivity diamond does not conduct.
 Purchase Standard Silicon Wafer At Online Wafer Manufacturing West Palm From pinterest.com
Purchase Standard Silicon Wafer At Online Wafer Manufacturing West Palm From pinterest.com
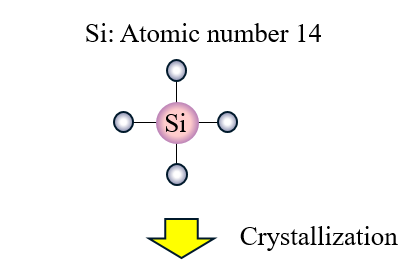
The resistivity data for silicon is widely available. Is a semi-conductor it is midway. The conductivity of silicon lies between the conductivity limits of conductors and insulators. Silicon is a chemical element with the symbol Si and atomic number 14. On the order of Kohm x m vs. Silicon is currently the most used material in space semiconductor devices.
Is a semi-conductor it is midway.
So it does conduct electricity. Silicon is currently the most used material in space semiconductor devices. Silicon is typically a very poor conductor of electricity and often considered an insulator. It is relatively unreactive. On the order of Kohm x m vs. Silicon is a chemical element with the symbol Si and atomic number 14.
 Source: pinterest.com
Source: pinterest.com
The remaining elements in period 3 do not conduct electricity. Silicon is a semiconductor meaning that it does conduct electricity. It was generally accepted that thin layers of silicon should not conduct electricity because a very thin surface does not have enough charge carriers and hence would not give an image. So it does conduct electricity. And germanium tin and lead are below it.
 Source: shindengen.com
Source: shindengen.com
It is a semiconductor so it is not a good conductor or a good insulator. Silicon is a chemical element with the symbol Si and atomic number 14. On the order of Kohm x m vs. In fact it has a resistivity of around 60000 ohm-cm compared with coppers 17e-6 ohm-c. The gap between these energy states and the nearest energy band is usually.
 Source: pinterest.com
Source: pinterest.com
Is silicon a conductor or an insulator. Silicon is a semiconductor Ie. Is a semi-conductor it is midway. It is a hard brittle crystalline solid with a blue-grey metallic lustre and is a tetravalent metalloid and semiconductorIt is a member of group 14 in the periodic table. It is a semiconductor so it is not a good conductor or a good insulator.
 Source: techiescientist.com
Source: techiescientist.com
Silicon dioxide does not conduct electricity under normal circumstances because in it no free electrons are present for conductivity. They have no free electrons that can move around and carry charge from place to. Silicon Carbide sometimes mistakenly referred to by the trade name Carborundum is used due to its properties of high hardness Mohs hardness 9 wear resistance its chemical inertness high thermal conductivity abrasion resistance low coefficient of thermal expansion thermal shock resistance and strength at high temperature ranges. Silicon is typically a very poor conductor of electricity and often considered an insulator. Phosphorus sulfur chlorine and argon.

Because of its high chemical affinity for oxygen it was not. Phosphorus sulfur chlorine and argon. Doping a semiconductor in a good crystal introduces allowed energy states within the band gap but very close to the energy band that corresponds to the dopant typeIn other words electron donor impurities create states near the conduction band while electron acceptor impurities create states near the valence band. The statement does NOT correctly compare silicon with another element is - Silicon conducts electricity as well as copper does. The negative electrons in the N-type silicon get attracted to the positive terminal of the battery.
 Source: co.pinterest.com
Source: co.pinterest.com
Is a semi-conductor it is midway. Phosphorus sulfur chlorine and argon. So it does conduct electricity. Doping a semiconductor in a good crystal introduces allowed energy states within the band gap but very close to the energy band that corresponds to the dopant typeIn other words electron donor impurities create states near the conduction band while electron acceptor impurities create states near the valence band. Although strictly classed as a semi-conductor pure silicon is closer to being an insulator than a conductor.
![]() Source: hbjinyong.com
Source: hbjinyong.com
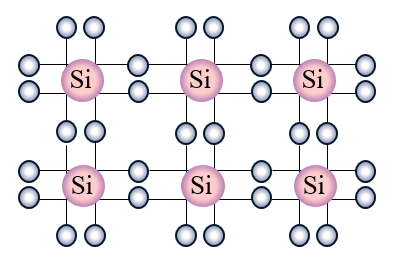
Variable electrical conductivity diamond does not conduct. Silicon is currently the most used material in space semiconductor devices. The resistivity data for silicon is widely available. It is relatively unreactive. Silicon has a giant covalent structure.
![]() Source: techiescientist.com
Source: techiescientist.com
Silicon has a giant covalent structure. It is relatively unreactive. The statement does NOT correctly compare silicon with another element is - Silicon conducts electricity as well as copper does. It is used as in insulator inside integrated circuits because it can be grown on the sio2 wafer by exposing it to steam. On the order of Kohm x m vs.
 Source: shindengen.com
Source: shindengen.com
It can conduct electricity as well as behave as an insulator by varying its properties. In fact it has a resistivity of around 60000 ohm-cm compared with coppers 17e-6 ohm-c. It is a hard brittle crystalline solid with a blue-grey metallic lustre and is a tetravalent metalloid and semiconductorIt is a member of group 14 in the periodic table. The remaining elements in period 3 do not conduct electricity. Variable electrical conductivity - diamond does not conduct.
 Source: pinterest.com
Source: pinterest.com
The positive holes in the P-type silicon get attracted to the negative. So it does conduct electricity. The positive holes in the P-type silicon get attracted to the negative. Silicon is a chemical element with the symbol Si and atomic number 14. The conductivity of silicon lies between the conductivity limits of conductors and insulators.
 Source: pinterest.com
Source: pinterest.com
Unlike a typical metal however silicon gets better at conducting electricity as the temperature increases. It was generally accepted that thin layers of silicon should not conduct electricity because a very thin surface does not have enough charge carriers and hence would not give an image. So it does conduct electricity. Silicon is a semiconductor Ie. They look metallic but conduct electricity only intermediately well.
![]() Source: electrical4u.com
Source: electrical4u.com
Carbon is above it. The negative electrons in the N-type silicon get attracted to the positive terminal of the battery. Electricity whereas graphite contains free electrons. It is a semiconductor so it is not a good conductor or a good insulator. The gap between these energy states and the nearest energy band is usually.
 Source: pinterest.com
Source: pinterest.com
Is silicon a conductor or an insulator. Electricity whereas graphite contains free electrons. Most house wiring is made of copper and not silicone because copper is a very good conductor of electricity. Even though N-type silicon by itself is a conductor and P-type silicon by itself is also a conductor the combination shown in the diagram does not conduct any electricity. The gap between these energy states and the nearest energy band is usually.
 Source: pinterest.com
Source: pinterest.com
On the order of Kohm x m vs. Because of its high chemical affinity for oxygen it was not. The negative electrons in the N-type silicon get attracted to the positive terminal of the battery. Silicon is a semiconductor Ie. Electricity whereas graphite contains free electrons.
 Source: quora.com
Source: quora.com
Silicon has a giant covalent structure. Silicon Carbide sometimes mistakenly referred to by the trade name Carborundum is used due to its properties of high hardness Mohs hardness 9 wear resistance its chemical inertness high thermal conductivity abrasion resistance low coefficient of thermal expansion thermal shock resistance and strength at high temperature ranges. They have no free electrons that can move around and carry charge from place to. On the order of Kohm x m vs. They all conduct electricity to some degree some more than others as a perfect insulator doesnt exist.

On the order of Kohm x m vs. Variable electrical conductivity diamond does not conduct. The remaining elements in period 3 do not conduct electricity. It is a hard brittle crystalline solid with a blue-grey metallic lustre and is a tetravalent metalloid and semiconductorIt is a member of group 14 in the periodic table. Is a semi-conductor it is midway.
 Source: pinterest.com
Source: pinterest.com
Most house wiring is made of copper and not silicone because copper is a very good conductor of electricity. Even though N-type silicon by itself is a conductor and P-type silicon by itself is also a conductor the combination shown in the diagram does not conduct any electricity. The grease does not conduct electricity so it shouldnt be applied directly to the mating surfaces pins and sockets of an electrical connection. It is a hard brittle crystalline solid with a blue-grey metallic lustre and is a tetravalent metalloid and semiconductorIt is a member of group 14 in the periodic table. Silicon is a semiconductor meaning that it does conduct electricity.
 Source: pinterest.com
Source: pinterest.com
Silicon Carbide sometimes mistakenly referred to by the trade name Carborundum is used due to its properties of high hardness Mohs hardness 9 wear resistance its chemical inertness high thermal conductivity abrasion resistance low coefficient of thermal expansion thermal shock resistance and strength at high temperature ranges. Nohm x m for copper. Most house wiring is made of copper and not silicone because copper is a very good conductor of electricity. On the order of Kohm x m vs. They all conduct electricity to some degree some more than others as a perfect insulator doesnt exist.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title does silicon conduct electricity by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





